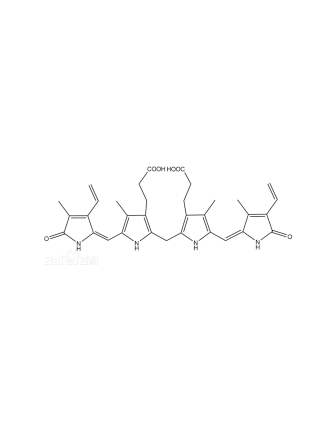
Bilirubin, also known as cholerythrin or bilirubin crimson, is the main pigment in animal bile and one of the primary components of gallstones. It is a linear structural compound containing four pyrrole rings linked by methylene groups at their α-positions, belonging to the class of diene bilins. Its molecular formula is C₃₃H₃₆N₄O₆, with a relative molecular weight of 584.7. Typically, it appears as pale orange or dark red-brown monoclinic crystals. Insoluble in water, it is soluble in benzene, chloroform, carbon disulfide, as well as acids and alkalis, and slightly soluble in ether and ethanol.
Bilirubin solids are relatively stable in a dry state, and its chloroform solution also maintains stability in the dark. However, it is highly unstable in alkaline solutions or when exposed to ferric ions (Fe³⁺), and is rapidly oxidized to biliverdin. When heated, bilirubin gradually darkens in color without melting, and its pale green solution emits a red fluorescence under ultraviolet light.
Bilirubin is mainly extracted from animal bile, with by-products such as bilirubin calcium salts and cholic acid obtainable during the extraction process. Based on its physical and chemical properties, various extraction methods have been developed, including the alcohol-free extraction method, concentration method, chromatography method, resin method, one-step method, and neutralization method, among others.
Bilirubin serves as an important reagent in analytical chemistry and biochemical research, and is also one of the raw materials for manufacturing artificial bezoar. In clinical medicine, it exhibits sedative, antispasmodic, antipyretic, hypotensive, and erythropoietic effects, and is used as a drug for the treatment of leukemia. In addition, bilirubin has strong inhibitory effects on Japanese encephalitis virus and cancer cells. It also possesses potent antioxidant properties, which can inhibit the occurrence of certain peroxidative damages in the body.